TL; DR
We mix experiments, simulations, and theoretical models to study how groups of cells interact with each other and their environment. We generally have two systems we study: bacterial biofilms and lab-evolved yeast. We use our background in physics to strive to find new and interesting phenomena in living systems. Three phrases to summarize our lab’s direction: “Aim for rough water”, “More is different”, “Interesting science is interesting”.
10,000 foot view
“Aim for rough water”. It is uncertain and, at times, terrifying, but also the most exciting. Everybody can see where they’re going in calm waters where there’s little to be discovered. Our research philosophy has been greatly influenced by the Nature essay providing such advice written by the late, great Steven Weinberg. In our interdisciplinary age, we no longer need to justify the utility of combining physics and biology. However, we are still in many cases, as a field, searching for the right questions to ask. We thus are able to discover fundamental phenomena – compared to other fields of physics, we are even still searching for our fundamental equations. We can “go for the messes” and lead emerging fields of study such as the evolution of multicellularity.
More is different. The titular message of Philip Anderson’s landmark paper published in Science, 1972, has also guided our research. With training in soft matter physics and colloidal particles, Peter’s guiding hypothesis is that you can’t understand what a group of particles is going to do by only studying a single particle. More particles is different. And if ‘dumb’, micron sized plastic particles exhibit complex emergent phenomena, then cells with similar physical descriptions are susceptible to similar emergent physical phenomenon. So, either group-level properties of cellular systems arise via similar soft matter physics, or these groups of cells do something to control and avoid the emergent effects. This leads us to experiment on, simulate, and attempt to theoretically model many cells interacting in relatively simple settings.
Interesting science is interesting. Ultimately, we are scientists with physics as our training. This shapes the way we create and attack problems. Biology, like everything else in the universe, must obey the laws of physics. But, given that fact, where is it useful to understand the physics in living systems? This mindset allows us to ask new and interesting questions. They may involve new physics or new biology, and interesting science is more than enough. We enjoy and welcome new and exciting collaborations that give us the opportunity to share our experimental techniques, modeling expertise, or just expand our interdisciplinary reach.
Microbial Interactions
Biofilms are surface attached communities of bacteria and other microbes. In other words, they are collections of micron-sized cells densely packed together. So, drawing on Peter’s colloidal particle background, we look at how both biological activity and physical behaviors can give rise to biofilm-level emergent properties. In particular, we enjoy figuring out which biological properties of interest can only be understood by diving into the physical properties.
The tools we have shape the kinds of questions we can answer. Typical studies of biofilms utilize fluorescence or confocal microscopy. We build on those techniques with mechanical testing using a universal testing machine (UTM), but especially with interferometry to study the three-dimensional surface profile, or topographies, of biofilms. Interferometry unlocks previously unavailable out-of-plane resolution of the surface which, turning back to soft matter physics, can carry a lot of information about what is happening beneath the surface. This gives us a much simpler mechanism to investigate biofilm behavior rather than having to stain particles and attempt to look deep inside the community.
Most of our biofilm studies incorporate questions and ideas about how cells contact and interact with each other. These interactions influence a range of properties from fundamental growth dynamics in three dimensions to cell arrangements in contact killing. Applying our physical understanding, we also investigate antibiotic susceptibility of microbes in these biofilms. The simplicity and versatility of interferometry will also allow us to expand beyond artificial, in vitro, settings such as agar plates or flow cells. Instead, our long-term goal is to extend our studies to more practical questions about biofilms where they actually form.
Origins of Multicellularity
How did the transformative transition to multicellularity occur dozens of times? The existence of multiple transitions implies that it is relatively easy. Yet, while phylogenetic studies have only been able to tell us how many times and when this transition has occurred, we know less about how it occurred. We hypothesize that physics provides a free scaffolding for multicellular development. Group level reproduction is an emergent consequence of interactions between cells.
Understanding how the group emerges as the Darwinian individual has been a sticking point in understanding the evolution of multicellularity. Extant, or continuing, multicellularity can assemble complex structures with complex functions due to their developmental genes. These genes can control gene expression spatially and temporally. Crucially, they can also be modified via mutation, and passed from parent to off-spring, enabling evolution to occur at the group level. Nascent, or emergent, multicellularity, however, faces a chicken or egg problem. Group level development is necessary, but development is required for multicellular adaptation, but development is itself a multicellular adaptation.
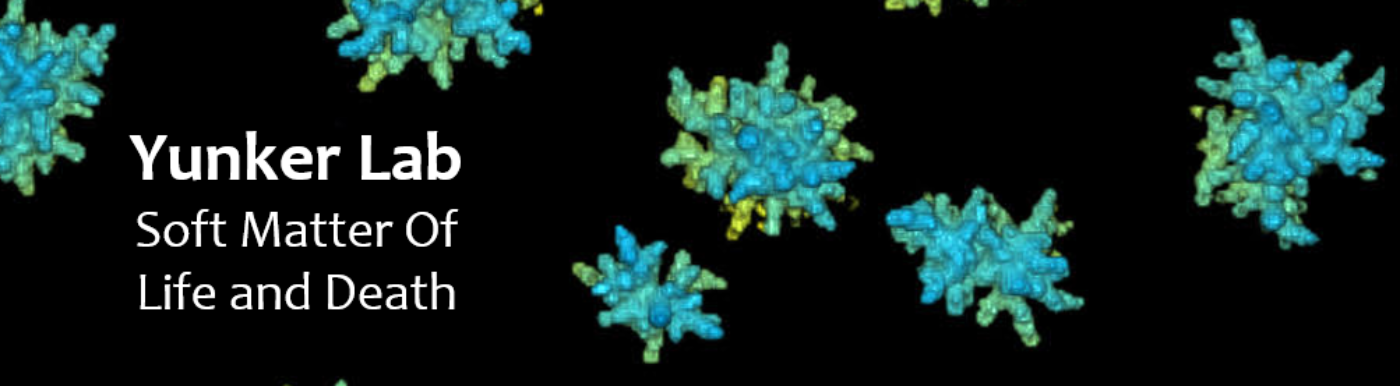
To address this problem, we study emergent group-level phenomena in Snowflake Yeast, a recently transitioned multicellular system from our collaborators. In this system, we have shown how mechanical stress breaks bonds which drives the need for a lower group packing fraction and thus elongated cells. The sufficiently random packing of these clusters creates a maximum entropy distribution of volume around each cell which is highly conserved from cluster to cluster. Therefore, any group trait relying on packing statistics will also be conserved.